When you savor a cup of decaffeinated coffee, have you ever wondered how caffeine is extracted from coffee beans? After conducting my own research, I began to ask myself other questions: Is decaffeinated coffee safe for consumption? What is the environmental impact of decaffeination methods? In this article, we will delve deep into the fascinating process of coffee decaffeination. Discover the various techniques used to remove caffeine while preserving the distinctive flavors and aromas of coffee. Get ready to uncover the secrets of decaffeination.
Malgré le processus de décaféination, il peut rester une petite quantité de caféine dans les boissons décaféinées. Selon une étude récente, ces boissons peuvent encore contenir entre 1 et 2% de leur teneur en caféine d’origine. Pour les cas les plus extrêmes, cela peut aller jusqu’à 20%.
Despite the decaffeination process, there may still be a small amount of caffeine in decaffeinated drinks. According to a recent study, these drinks can still contain between 1 and 2% of their original caffeine content. In extreme cases, this can go up to 20%.
A little history about the beginning of coffee decaffeination
The chemist Friedlieb Ferdinand Runge succeeded in extracting pure caffeine from coffee in 1820. However, he did not patent his process to commercialize decaffeinated coffee. In reality, he was mainly interested in studying this molecule. However, he did not make great strides in understanding its chemical properties.

The first commercial decaffeination process was invented by the German merchant Ludwig Roselius in 1903 (patented in 1906). Ludwig discovered this method by chance when his cargo of beans was soaked in seawater during an entire voyage. His coffee had lost much of its caffeine without losing much flavor.
“As a patron, he supported several leading artists, such as Paula Modersohn-Becker or Bernhard Hoetger.”
https://fr.wikipedia.org/wiki/Ludwig_Roselius
Ludwig discovered this method by chance when his cargo of beans was soaked in seawater during an entire voyage. However, this process is no longer used due to the carcinogenic properties of benzene. Since then, other less toxic methods have been developed to extract caffeine from coffee beans.
The shared characteristics of decaffeination methods
In all decaffeination processes, coffee beans are decaffeinated before being roasted. The main challenge of decaffeination is to separate caffeine from coffee beans while preserving other chemical compounds at their original concentrations. This proves to be a complex task. Coffee contains a large number of chemical compounds that contribute to its taste and distinctive aroma.

Caffeine is a hydrophilic polar substance, which means that water is used in all decaffeination methods. However, water is not the ideal solvent for decaffeination. It is not selective and also removes other soluble substances. That’s why most decaffeination processes use decaffeinating agents. These agents can be methylene chloride, activated carbon, CO2, or ethyl acetate, to obtain a more precise result.
The Different Methods of Decaffeinating Coffee
There are several methods to decaffeinate coffee, all of which are performed on unroasted coffee beans. The beans are first steamed and then rinsed with a solvent that extracts caffeine while preserving other components. This process is repeated 8 to 12 times until the caffeine content reaches the required standards, which is a 97% elimination of caffeine according to the American standard and a 99.9% mass elimination according to the EU standard.
Decaffeination methods using organic solvents
Solvents used in decaffeination
Due to health concerns, the first solvents used for decaffeination were replaced by dichloromethane and ethyl acetate. Dichloromethane, which is used in the United States, is capable of selectively extracting caffeine and has a low boiling point. However, it is slightly toxic and carcinogenic, which is why the amount of residual solvent must be less than 10 parts per million (ppm). Ethyl acetate, which is also slightly toxic, subsequently replaced dichloromethane.

Curiously, coffee decaffeinated with this solvent is sometimes marketed as “naturally decaffeinated” because ethyl acetate can be obtained from a biological process such as sugar cane fermentation.
https://www.maisonducafe.com/cafes/decafeination/
Decaffeinate with supercritical fluid
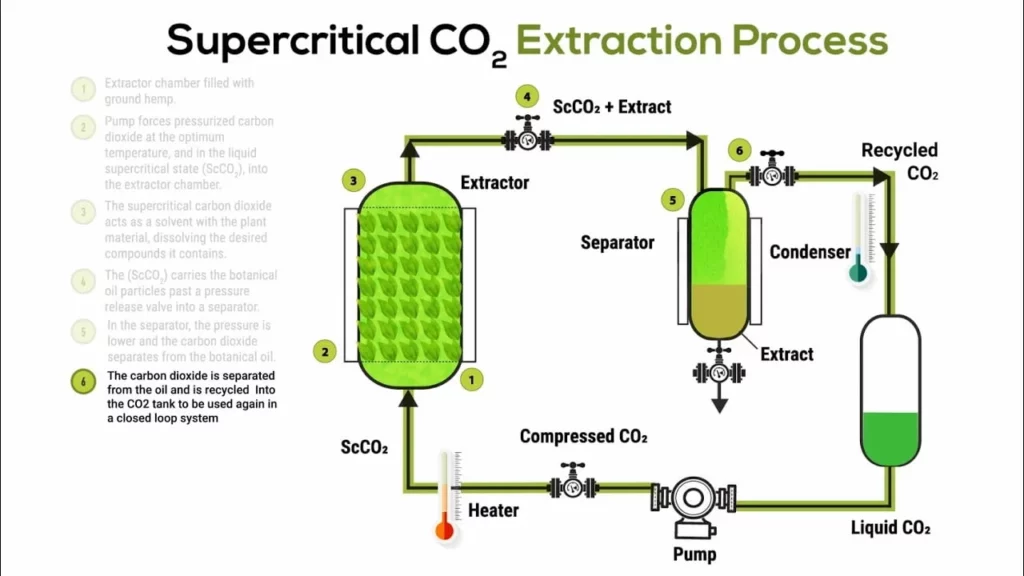
Supercritical fluid extraction uses CO2 in a supercritical state to selectively act on caffeine, releasing only this alkaloid. Water-soaked coffee beans are placed in a sealed extraction vessel, where supercritical CO2 passes through the coffee at high pressures to extract caffeine. CO2 acts as a solvent to dissolve and remove caffeine from coffee beans while preserving larger aromatic components.
Caffeine-laden CO2 is then transferred to an absorption chamber where pressure is released, allowing CO2 to return to its gaseous state and leave caffeine behind. Caffeine is removed using charcoal filters, and CO2 is recycled. This process has the advantage of avoiding the use of potentially harmful substances and produces commercially available decaffeinated coffees that are less exotic and available in grocery stores.
In this method, coffee beans are soaked in hot water containing other components that contribute to the taste of coffee. After about 10 hours of treatment, the water is filtered through activated charcoal that retains caffeine. The water is then brought back into contact with the coffee and evaporated, leaving behind a coffee with a good aroma.
Find our history articles here.

Leave a Reply